Development of Liquid Analyzers
The principle
In case of spectroscopy of liquids, the absorption lines are strongly perturbed, so that the simple Beer-Lambert law is not suitable any more. For instance there is no effect due to pressure. Few data are available in literature, and they are usually sparce. The same molecule can be dissolved in different solvents, with different sample lengths, so very often the first approach requires a do-it-yourself analysis of the target species.
On the other side, when dealing with heavy molecules, for which the absorptions are grouped in wide continuous bands (~10 cm-1), rather than in combs of lines (~1 GHz), measurements in the liquid phase do not alter too much the spectra, so providing alternative experimental arrangements.
The absorptions of the solvent must be taken into account, of course.
Dioxins and furans
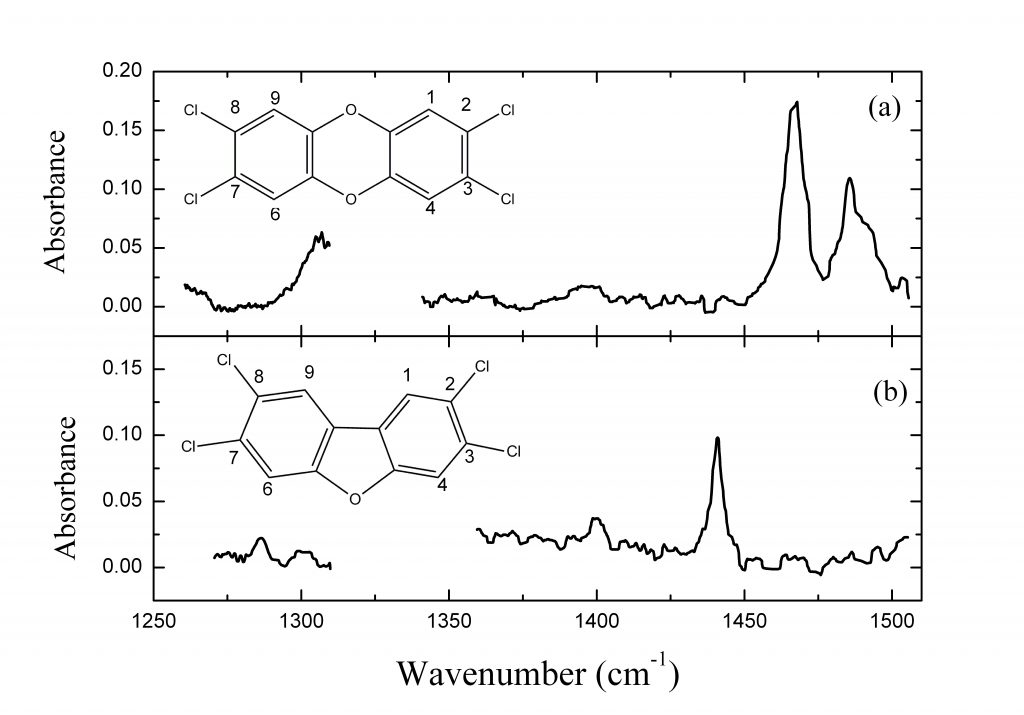
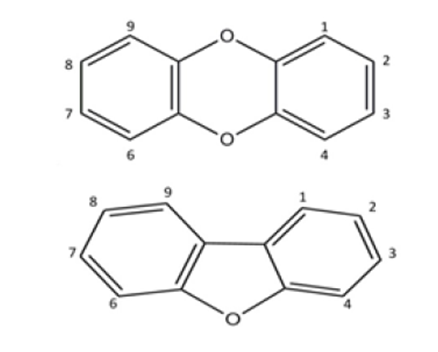
In the frame of the SIMPAS Project, we have studied most of the 17 toxic species of dioxins and furans. The general structures of these molecules are shown on the right. Their toxicity depends on the number and positions of the chlorine atoms, in the locations labelled with the numbers 1-4, 6-9.
Dioxins and furans feature strong (and wide) absorptions in the middle infrared, where widely tunable quantum cascade lasers are now available. By sweeping the laser wavelength it is possible to record the absorption spectra of the samples, so allowing the reconstruction of its composition (at least to 15 different species, we didn’t push the method further).
The experimental apparatus is shown below. Two QCLs, covering the ranges 1205-1310 and 1335-1520 cm-1, are alternately sent along the same optical path, across an absorption cell, 2 mm long, filled of the proper sample of dioxins/furans dissolved in CCl4.

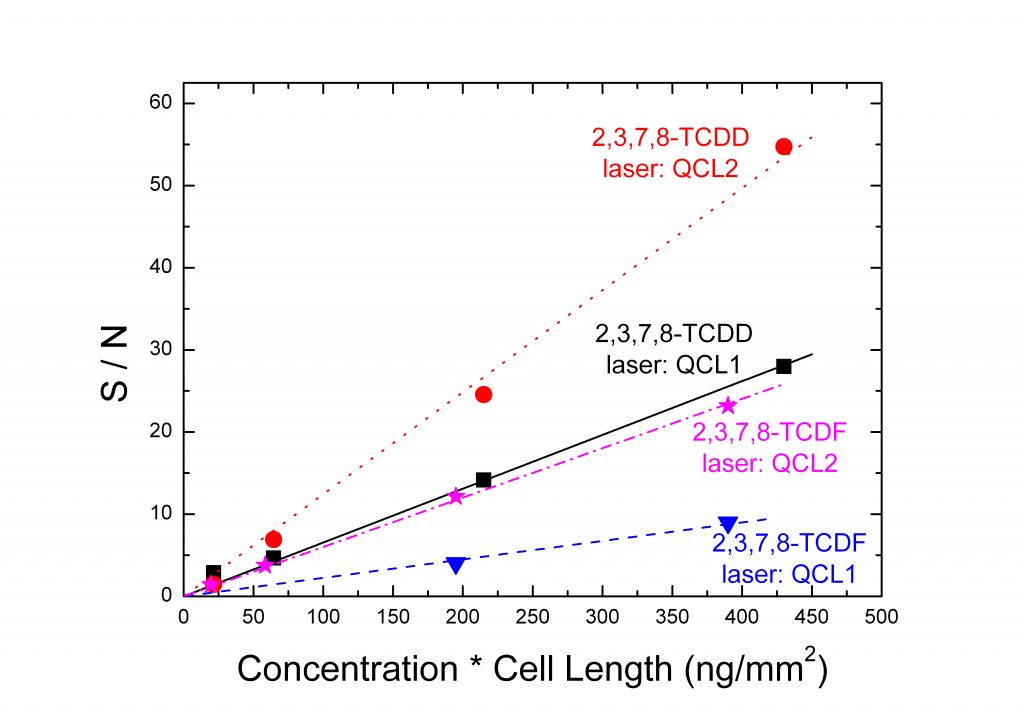
The figures below are an example of spectra (Top) and a graph of the S/N as a function of the concentration in the sample of the most toxic dioxin and furan (Bottom).